Ken writes:
I’ve been reading about beer filtration for a test this afternoon. Filtration is one the last steps before beer is packaged and put out to sale. It’s a near universal practice but deprecated by beer aficionados. On the whole, I’ve been in the ‘anti’ camp on the subject but I’ve been forced to think it through a little more carefully.
Beer filtration has one intended purpose, which is to remove particulate matter from the beer so that it looks clear in the glass. The particles can be yeast cells, protein flocs, protein-polyphenol complexes or anything else that might be floating in there. But I’ve always viewed filtration with prejudice because I thought it removed some of the flavour of the beer along with all those other things.
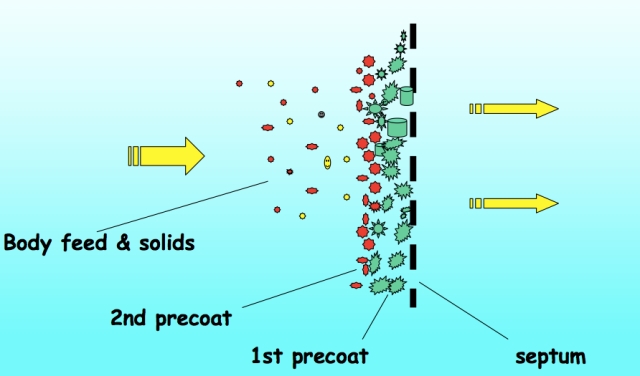
There are two basic filtration mechanisms: sieving and depth-plus-adsorption. Sieving means that the filter has tiny pores in it and only molecules smaller than the pores can pass through the filter. Large material is kept back. The Depth-plus-adsorption approach is different. Here the filtering is achieved by a combination of a large pored backing sheet and a build up of fine powder held in place by the sheet. The powder, usually a substance called Kieselguhr or diatomaceous earth, forms a deep bed which the beer has to pass through. As it passes through, the flocs and yeast and other particles in the beer are adsorbed onto the bed and held there. Both approaches result in very clear beer.
A schematic picture of the depth plus adsorption approach and a close up image of the diatomaceous earth that is used as a filter bed.
As I said, I used to think that filters strip flavour out. But I now see that this is cannot be right. What are the flavours in beer? They are flavour active chemicals like acetaldehyde (not pleasant in large quantities, grassy, or green apples), ethyl acetate (fruity, solvent), isoamylacetate (peardrop, banana) and so on. But none of these are very large molecules. I don’t know how many Daltons they are, but probably not more than a couple of hundred Daltons each. They are round about the size of the various sugar molecules.
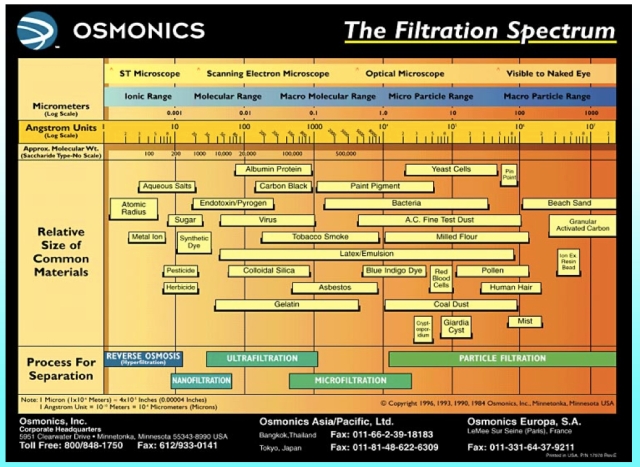
The chart below gives an indication of the size of different kinds of substance and the types of filtration applicable to those sizes.
Bacteria are around 1-4 micrometers (millionths of a meter), yeast are around 4-8. Large protein molecules are smaller still, between a tenth and half a micrometer. But the flavour important compounds in beer are absolutely tiny. This chart puts molecules with a molecular weight of 200 at around 0.001 micrometers (i.e. a billionth of a meter). Ethyl acetate has a molecular weight of 88. Isoamylacetate is 130. Acetaldehyde is 44. In other words, they are easily small enough to pass through the filter bed. So if the filter strips the flavour out of beer there must be some other explanation.
On the other hand, filtered beer and beer fresh from the conditioning tank manifestly taste differently. If you have the opportunity to try a cask beer and a filtered/carbonated version of the same side by side, it’s apparent that they do taste differently. So what is the explanation?
I think there are two; both involving yeast. Some of the main favour active chemicals in beer are isomerised hop alpha acids (iso-α-humulone, iso-α-cohumulone, and iso-α-adhumulone). These chemicals can stick to yeast. So that when the yeast is filtered out, the resulting beer is less bitter than it was with the yeast still present. So, if you want to brew a bitter beer that will be filtered, you need to correspondingly increase the amount of iso-α-acids in the beer.
The second reason I can think of why filtered beer won’t taste quite like unfiltered beer is that it is a step that can allow dissolved oxygen (DO2) pick up in the beer. DO2 can be sucked through the pump casing into the pump impeller during pumping. Dosing diatomaceous earth into the beer on its way to the filter can draw in DO2. The filter casing itself will have air in it unless it has been thoroughly flushed with deaerated water. Put simply, every time the beer is moved it is at risk of exposure to oxygen. In unfiltered beer this is not a problem because yeast mops up oxygen and uses it to grow, so the oxygen cannot react negatively with compounds in the beer to produce stale tasting flavours. Filtering out the yeast removes the best defence against beer oxidisation. If you filter beer, your dissolved oxygen control has to be absolutely flawless. It is really only the largest breweries with the best technology that can pull this off.


It sounds a reasonable explanation to me. What do other brewers think of it?
I’m fairly confident they would agree. Especially with the last paragraph on the dangers of too much contact with oxygen.
An here I thought you were just over there to drink. Look at you with your polymerases! Interesting stuff, I’m looking forward to more brewing related posts.
i’ve already–or should that be ALEready–written a couple. If you follow the beer category, you should be able to find them.